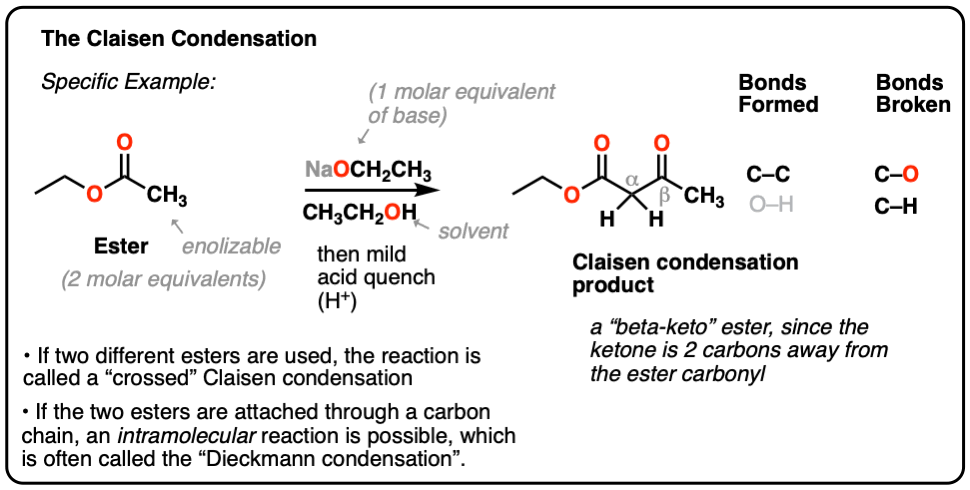
A major difference with the aldol reaction is the fact that hydroxide cannot be used as a base because it could possibly react with the ester. We can make alpha-protons more easily-removable by using a beta-keto-ester.
 Claisen Condensation And Dieckmann Condensation
Claisen Condensation And Dieckmann Condensation
Organische Chemie 1971 2 3 no-no.

Beta keto ester. Gamma-keto acids Gamma-ketoacids or 4-oxoacids such as levulinic acid have the ketone group at the third carbon from the carboxylic acid. Not being bound to salt means Esters can contain more Beta-Hydroxybutyrate BHB. This protocol offers mild reaction conditions and a broad substrate scope.
The Claisen condensation is a carboncarbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base resulting in a β-keto ester or a β- diketone. Keto-Ester gibt Power hat aber keine aufputschende Wirkung wie zum Beispiel Koffein. Chem 2018 83 14834-14841.
Angewandte Chemie International Edition 2013 52 37 9763-9766. BHB is one of the three ketones bodies that we produce. ALKYLIERUNG DER DIANIONEN VON BETA-KETOESTERN.
They can be formed by the Claisen condensation. An one-pot reaction of carboxylic acids and ynol ethers provides β-keto esters under promotion of Ag 2 O and a subsequent DMAP-catalyzed rearrangement. The reaction of alkyl lithium compounds with β-keto esters.
Salts are bound to Sodium Potassium or Calcium. 13-Diketones add oxidatively to alkenes in a reaction mediated by CAN to give dihydrofurans Equation 101. The product is a β-keto ester.
β-Ketoesters react with 12- and 13-dielectrophiles to give furan-3-ones and dihydropyranones respectively. Gottfried Brieger Danel G. These are substances that contain ketones and lead to the state of ketosis.
Access to β-Keto Esters by Palladium-Catalyzed Carbonylative Coupling of Aryl Halides with Monoester Potassium Malonates. Instead an alkoxide version of the alcohol used to synthesize the ester is used to prevent transesterification side products. However esters differ from ketone salts since theyre formed by binding an alcohol molecule to a ketone body.
The regio- and stereoselective aminochlorination of αβ-unsaturated ketones with NN -dichloro- p -toluenesulfonamide 4-TsNCl 2 and CuOTf as catalyst provides an easy access to vicinal haloamino ketones with excellent regioselectivity and good yields. During the reaction a new carbon-carbon bond is formed. It is named after Rainer Ludwig Claisen.
Ketone Ester C8H16O4 CID 44631890 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Beta-keto acids Beta-ketoacids or 3-oxoacids such as acetoacetic acid have the ketone group at the second carbon from the carboxylic acid. If there are two alpha-protons they can both be removed and replaced with R fro.
Manganese triacetate can also be used as the oxidant. Es handelt sich dabei um reine Beta-Hydroxybuttersäure die auch vom Körper selbst hergestellt wird wenn wir fasten oder extrem hungern. It is present in.
